Timeline: Hydrothermal vent research
At deep-sea hydrothermal vents, entire ecosystems thrive in complete darkness. At the beginning of their food chain are chemical energy carriers such as molecular hydrogen. Since their discovery, hydrothermal vents have been discussed in connection with the origin of life because the Earth provides chemical energy there. The chemical conditions at hydrothermal vents are conducive to the conversion of CO2 into organic substances – the building blocks of life. Was such chemistry at the origin of life?
In 1977, a group of marine geologists around Jack Corliss (born 1936) and Tjeerd van Andel (1923–2010) on an expedition discovered geothermally active hydrothermal vents on the ocean floor in the Galápagos Rift around the Galápagos Islands. The scientists were on the research submarine DSV Alvin operated by the Woods Hole Oceanographic Institution (WHOI). The geysers discovered in the deep sea emit iron and sulfur minerals, as well as gases such as methane and hydrogen sulfide. Samples of the vents were taken during the dive. A dense colonization of bacteria and archaea as well as large worms was found – a thriving ecosystem far from the sunlight. Since then, biologists have suspected that life evolved around such hydrothermal vents because they provide energy and nutrients and are protected from natural disasters on the planet‘s surface.


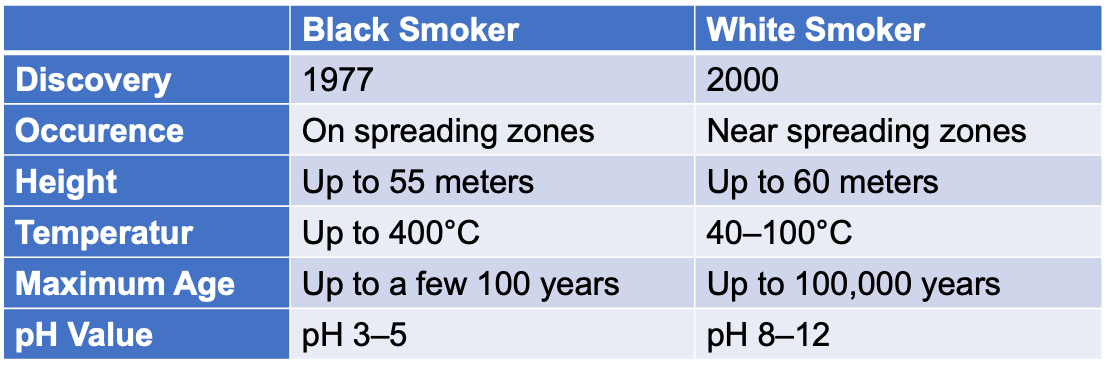
The hydrothermal vents first discovered were known as Black Smokers. They shelter highly diversified ecosystems whose energy source largely comes from volcanism on the sea floor. Their vents show the picture of a primordial habitat on the early Earth, with reactive gases, dissolved transition metals, and thermal and chemical gradients. The Black Smokers usually lie directly above the spreading zones on the mid-ocean ridge. Where the continents drift apart, there is a magma chamber about 1–3 km below the sea floor. Through cracks in the sea floor near the spreading zone, cold seawater flows for kilometers down into the Earth‘s crust, where it comes in close proximity to the magma chamber (circa 800–1200 °C). The heated water then rises via convection and re-enters the sea at the Black Smoker at a temperature of up to 400 °C. The exiting water is not only very hot, but also very acidic (pH 2–3) and contains large amounts of dissolved metals such as iron and manganese, as well as hydrogen (H2), carbon dioxide (CO2), hydrogen sulfide (H2S) and methane (CH4). This creates the impression of a cloud of black smoke billowing out of tubelike structures. The dissolved gases form the basis of life for microbial communities that are at the beginning of the food chain in Black Smoker ecosystems.
Soon after their discovery, they were specifically discussed by the American biologist John Baross (born 1940) in connection with the origin of life, because these systems offer extremely reactive geochemical conditions, an important prerequisite for prebiotic syntheses. However, supporters of the primordial soup theory remained extremely skeptical that hydrothermal vents could be relevant to the origin of life at all. After all, the water at the exit point of Black Smokers is up to 400 °C hot, while the temperature record for microbial growth is „only“ 121 °C.
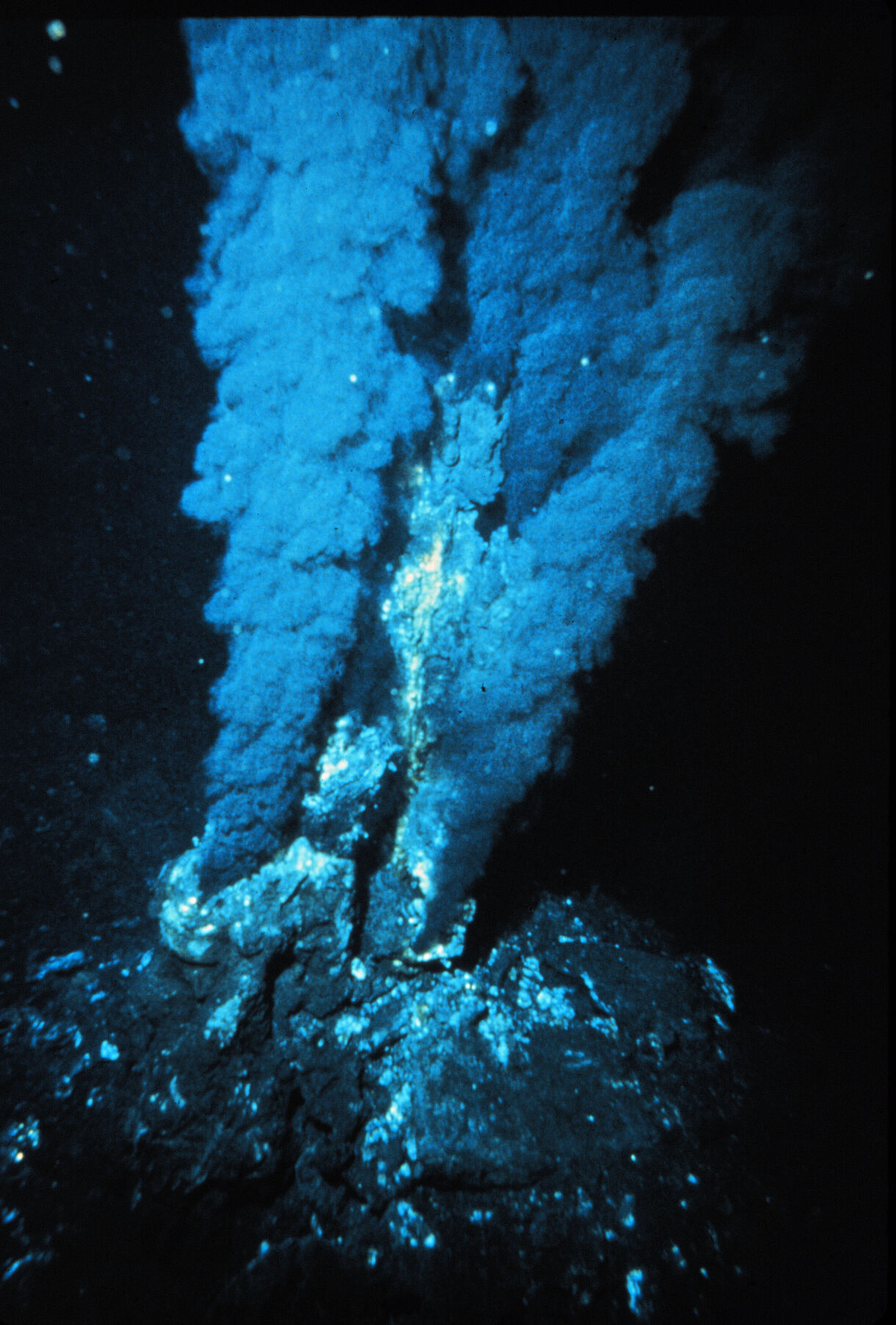
Soon after the discovery of the first hydrothermal vents, they were specifically discussed in connection with the origin of life, because these systems offer extremely reactive geochemical conditions, an important prerequisite for prebiotic syntheses. John Baross (born 1940), Jack Corliss (born 1936) and Sarah E. Hoffman were the first researchers to hypothesize a relationship between hydrothermal vents and the origin of life. They suggested that a mixture of metal-containing minerals in contact with gases like carbon dioxide and ammonia would result in prebiotically important building blocks. However, supporters of the primordial soup theory remained skeptical that hydrothermal vents could be relevant to the origin of life at all. After all, the water at the exit point of Black Smokers is up to 400 °C hot, while the temperature record for microbial growth is „only“ 121 °C.

Also, the chemist Günter Wächtershäuser (born 1938), in a series of articles between 1988 and 1992, suggests that the earliest life form came from a volcanic hydrothermal field at high pressure and temperature. He hypothesized that early life may have formed on the surface of iron-sulfur minerals and coined the term iron-sulfur world. He linked the formation of complex biomolecules to a continuously available and reliable energy supply. The energy comes from the reduction of sulfur in iron-sulfur minerals such as pyrite (FeS2) with elemental hydrogen (FeS2 + H2 ⇌ FeS + H2S). The reaction provides enough energy to drive a prebiotic ammonia synthesis and also a synthesis reaction for building blocks of biomolecules and their polymerization. His idea is that once a primitive autocatalytic metabolism was established, a chemistry was set in motion that yielded ever more complex organic compounds, more and more complex metabolic pathways, and more and more complex catalytic centers.

The submarine hot hydrothermal vents were discovered less than 50 years ago. Since their discovery, people have considered the idea, as one alternative to a prebiotic soup. This idea is often dismissed by its critics on the grounds that submarine hydrothermal vents are simply too hot to have had anything to do with the origin of life. Although less warm hydrothermal vents have existed for at least 30 000 years, they were still unknown in the 1990s. Even before they were discovered, Michael J. Russell (born 1939) pondered the origin of life in iron sulphide bubbles at warm, alkaline submarine vents. He wrote about this in his 1994 article A hydrothermally precipitating catalytic iron sulphide membrane as a first step toward life (Journal of Molecular Evolution 39, 231–243):
»We propose that life emerged from growing aggregates of iron sulphide bubbles containing alkaline and highly reduced hydrothermal solution. These bubbles were inflated hydrostatically at sulphidic submarine sited some distance from oceanic spreading centers four billion years ago.« (S. 231)
The origin of life at Black Smoker-type hydrothermal vents has been viewed with skepticism by many critics because the environment there is too hot. While it‘s true that life couldn‘t exist at around 400 °C, it‘s not true that all hydrothermal vents are that hot. Some hydrothermal vents are much cooler than the famous Black Smokers originally discovered. Such warm, alkaline, deep-sea hydrothermal vents were discovered in 2000 by Deborah Kelley (born 1958) and her colleagues. The exiting water of which has a temperature of around 40–90 °C.
These hydrothermal vents are located in a hydrothermal field called Lost City. It is located at a depth of about 800 meters in the Atlantis massif, a mountain range at the bottom of the mid-Atlantic. The first representative of this class was called Lost City. Systems like Lost City have very favorable properties when it comes to the origin of life. A very important difference between the Lost City system and black smokers is that Lost City is located a few kilometers from the spreading zone – with the result that the hydrothermal water also circulates kilometers deep through the Earth‘s crust in a convection current, but not in contact with a magma chamber. In the Earth‘s crust, the descending seawater is heated to around 150–200 °C, but when it re-enters the sea, its temperature is only around 40–90 °C. Another important difference is that the hydrothermal water at Lost City flow through rock that is very high in magnesium and iron and very low in silica. As a result, the water leaving Lost City is very alkaline (pH 9–11). Unlike Black Smokers, who tend to be short-lived, Lost City has been active for around 100,000 years. The spilled water from Lost City contains a lot of hydrogen (H2), methane (CH4), and other short-chain hydrocarbons, but almost no carbon dioxide (CO2). Lost City type hydrothermal vents offer in many ways a conceptual alternative to the primordial soup. They even catalyze chemical reactions that show striking similarities to the main energy-yielding reactions of some microorganisms.



Since the discovery of hydrothermal vents, they have been discussed in connection with the origin of life. In submarine hydrothermal vents, precipitation membranes grow at the interface between seawater and mineral-rich fluid flowing from the vent, forming the characteristic vents. Such membranes are increasingly being ascribed a crucial role in the origin of life, but their bioenergetics are unclear. Therefore, some scientists use the so-called chemical gardens to gain clarity about the origin of life at hydrothermal vents.
The Chemical Garden is a fascinating chemistry experiment. The gardens form when metal salt crystals, such as ferric chloride, come into contact with water glass — a syrupy solution of sodium silicate — or other solutions containing certain anions. The twisted precipitates sprouting from the crystal are porous structures. The chimney-like tubes rupture and reform when the water flowing through them puts pressure on them. If such a vent existed in the ancient (presumably acidic) ocean, a gradient would have formed at the vent-ocean interface. Gradients are full of potential energy. In living cells, gradients are as conserved as the genetic code itself. Biology uses the flow of ions across a cell membrane to fuel the production of the energy carrier adenosine triphosphate (ATP), a most versatile fuel. Life requires an intermediary for this particular process (a protein), but Lost City‘s chemistry may have driven the reaction.


The question of the origin of methane, hydrogen and the short hydrocarbons in Lost City‘s spilled water leads us to an extremely important and interesting process that could be highly relevant to the origin of life: serpentinization. Serpentinization is a series of geochemical reactions in the Earth‘s crust initiated by hydrothermal systems. The process is named after the mineral serpentinite that is formed in the process. During serpentinization, hydrogen comes from interactions between water and rock in the Earth’s crust. Water is drawn into cracks in the Earth’s crust (by gravity) to a depth of a few kilometers. If we summarize it to the essentials, part of the iron (Fe2+) in the rock olivine is oxidized by water (to Fe3+) as is present in the magnetite rock. This oxidation leaves Fe3+ with one less electron than Fe2+, meaning that something has to be reduced. Where do the electrons go during serpentinization? They produce hydrogen from water, which is dissolved as a gas in the hydrothermal water and carried into the sea. Some electrons can also go to carbon dioxide (CO2) to create organic compounds and methane. This process of organic synthesis within the Earth is very interesting in the context of early life. Hydrogen is still a vital form of chemical energy for many microbes today.
Serpentinization also occurs in the hydrothermal systems beneath Black Smokers, but our focus for the origin of life remains with Lost City because these are alkaline and not too hot. The alkaline pH is the result of the process of serpentinization.




The rock beneath the hydrothermal vents in Lost City is mostly serpentinite. This occurs during the geochemical process of serpentinization in the Earth‘s crust. A by-product is molecular hydrogen (H2), but carbon dioxide (CO2) and methane (CH4) can also form. The H2 coming from the serpentinization continuously reduces CO2. CO2 reduction is at the beginning of all ecosystems, because life consists of reduced carbon compounds, all of which ultimately come from CO2. The geochemical reactions at Lost City may provide important clues to the transition from geochemical processes to biochemical evolution in the origin of life. Today there are only two groups of organisms that introduce CO2 into the global biological carbon cycle: photoautotrophs and chemoautotrophs. The former use the energy of sunlight to fix CO2. The latter use purely chemical energy, for example in the form of H2, which serves as a central electron donor in the metabolism of many prokaryotes. Here, anaerobic energy-yielding metabolic reactions (= reactions without the participation of oxygen) of microbes (methanogens and acetogens) in which H2 is involved as an electron donor stand out because they synthesize energy by reducing CO2. There are striking parallels between the chemistry of the H2/CO2 couple found in hydrothermal vents and the energy-yielding metabolic reactions of some modern prokaryotic organisms. The biochemistry of these microbes, in turn, could hold clues to the type of reactions that initiated the chemistry of life. This idea became more and more concrete through the collaboration of William F. Martin (born 1957) from the Heinrich Heine University in Düsseldorf and Michael J. Russell (born 1939). Martin‘s views on the central role of hydrogen in carbon metabolism fitted into the geochemical environment provided by alkaline hydrothermal vents. The geochemical approach from carbon dioxide and hydrogen to simple organic molecules and the biological approach, looking at molecular evolution in cells, led to the same conclusion: Hydrothermal vents bring together geology and microbiology to bring new life to the exploration of one of the most important questions in biology – what is the origin of life? Could modern metabolic pathways be “geochemical fossils”?

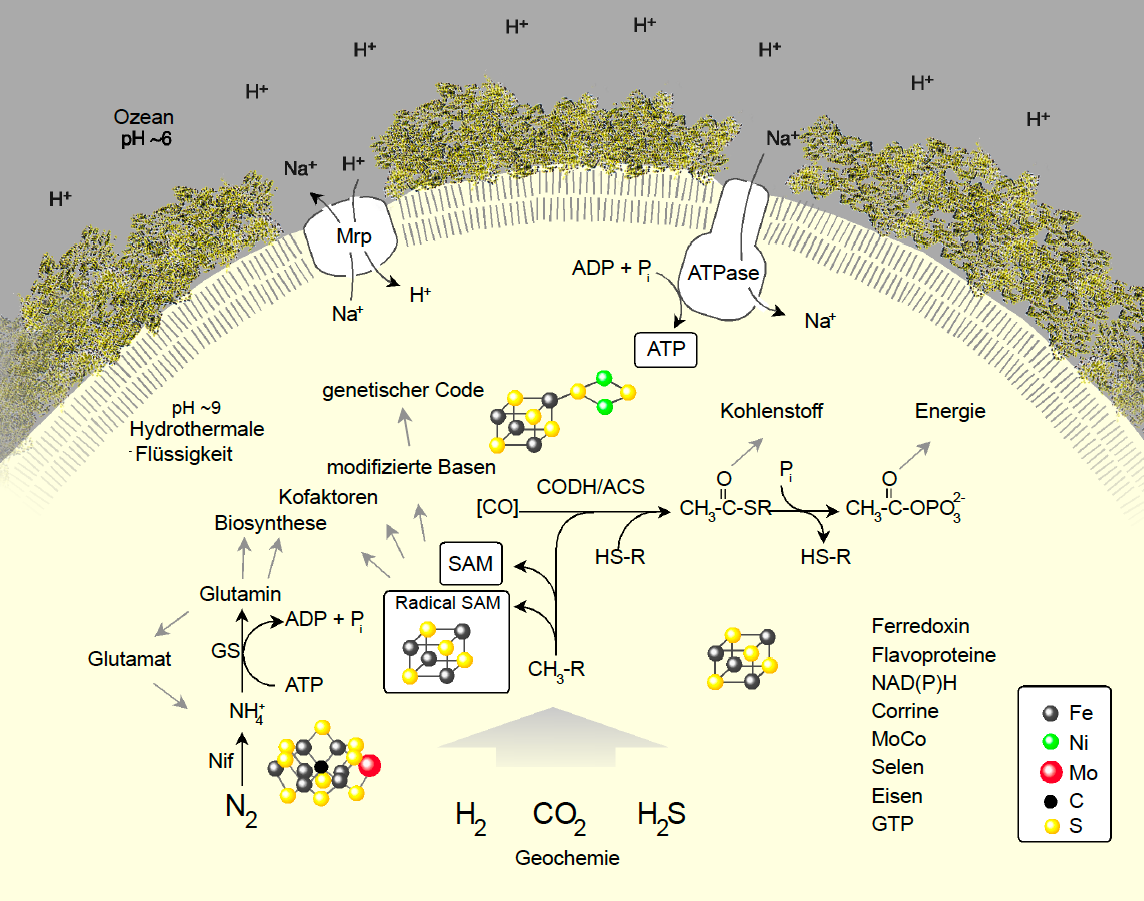
All available geochemical, microbiological, chemical, physical and bioinformatic information on hydrothermal vents and primordial microbes made it possible for William F. Martin (born 1957) from the Heinrich Heine University Düsseldorf to generate a picture of the origin of life at alkaline hydrothermal vents. The chemistry relevant to the origin of life took place at the bottom of the ocean in the alkaline hydrothermal vents, the white smokers. The circulating water at the white smoker hydrothermal vents never sees magma and is below 100 °C. Under these conditions the process of serpentinization takes place, which is very important for the origin of life because it produces chemical energy in the form of hydrogen gas. This hydrogen is the source of energy and electrons at the origin of life. The hydrogen can react with the surface of metals and minerals produced in the hydrothermal vents. On these surfaces, hydrogen meets carbon dioxide and the reaction of life takes place. The metals also activate carbon, oxygen, and nitrogen, which can react with each other on the solid surface. Because these reactions release energy, there is nothing stopping them from happening spontaneously. The products of the reactions can continue to react and the backbone of the microbial metabolism unfolds naturally. The more the compounds react, the more stable the products become. The stable products can accumulate on the surfaces of the cavities in the hydrothermal vents, especially in cooler regions, increasing their local concentration and promoting the build-up of more complex molecules. The products of organic synthesis can themselves be catalytically active. When products catalyze reactions that flow back into their own synthesis, autocatalytic networks can form. Autocatalytic networks self-organize, bridging a gap between chemical reactions and living cells. Continuously driven by energy-releasing reactions of H2 and CO2, transitions to higher molecular complexity become possible. Small peptides (= short amino acid chains, proteins) and nucleic acids (like RNA and DNA) can accumulate. This is the basis of the genetic code that directs the synthesis of proteins from RNA templates. This creates highly efficient catalysts and rapid acceleration of molecular organization. However, precursors must be constantly added throughout the process and the system remains dependent on H2 and CO2. Some hydrothermal vent cavities collect enough information to incorporate inorganic catalysts into genetically encoded proteins. The reaction networks can become free living cells, but their impulse remains dependent on the reaction of hydrogen and carbon dioxide. The first bacteria and archaea according to this theory were acetogens and methanogens, respectively. Organisms that live on H2 and CO2 and still inhabit the Earth‘s crust today. This film clearly explains this theory of the origin of life at alkaline hydrothermal vents.


With increasing information about the geological conditions at hydrothermal vents and advances in microbiology and bioinformatics, it has become possible to merge geology with microbiology and reconstruct the Last Universal Common Ancestor (LUCA). LUCA therefore plays a central role in studies of early evolution and the origin of life, connecting the abiotic phase of Earth‘s history with the first geochemical traces of microbial life around 4 billion years ago. LUCA has long been considered the common ancestor of microbes (bacteria, archaea) and eukaryotes. However, new genealogies of life show the origin of eukaryotes within the archaea, making LUCA the common ancestor of bacteria and archaea. Until 2016 there was no information where and what LUCA lived on. In 2016, William F. Martin and his team were able to identify 355 genes that provide information about LUCA‘s way of life and habitat. The genes were determined using bioinformatic analyses of microbial genome data. The genes indicate that the first cells likely lived in an environment very similar to today‘s deep-sea hydrothermal vents: A geochemically active environment rich in H2, CO2 and iron. LUCA was probably anaerobic (lived without oxygen), warmth-loving (thermophilic) and lived on gases: hydrogen (H2), carbon dioxide (CO2), carbon monoxide (CO) and nitrogen (N2). LUCA‘s proteins were richly endowed with iron-sulfur centers and required partners (cofactors) in which transition metals such as iron, nickel, and molybdenum play a key role.
Although LUCA is only a theoretical construct that was a „half-alive“ cell living in the small compartments of a vent system from alkaline hydrothermal vents on the geochemistry provided, this microbial ecology closely resembles that of modern-day acetogenic bacteria and methanogenic archaea, the physiologically primordial microbes.



The first living cells on Earth lived from chemical energy. Everything needed to thrive was present in rocks, metals, carbon dioxide (CO2), water and the hydrogen (H2) from hydrothermal activity. Neither sunlight nor ultraviolet light (UV) was required to awaken or keep the first cells alive. It follows that in the search for more life in our solar system, light does not have to be a limiting factor. Therefore, when searching for extraterrestrial life, distant moons like Enceladus become the focus of interest: It orbits the planet Saturn. It has a liquid ocean of water under its icy surface, its core is stony and metal-rich, and very high hydrothermal activity has been found at its south pole. Such a chemical environment could in principle support the emergence of life. Whether future missions to Enceladus provide evidence for the existence of complex chemical reactions, or even find molecular building blocks of life, remains to be seen. It‘s entirely possible that at least one interesting rock-water-carbon chemistry is happening there in total darkness, under the ice of a distant moon.
On April 14, 2023, the ESA „Juice“ mission was launched to the planet Jupiter, more than 600 million kilometers away. She is to investigate whether there are habitats under kilometer-thick ice crusts on the three largest icy moons of the planet (Europa, Ganymede and Callisto). Although the moon Europa is significantly smaller than the Earth, it has ocean layers larger than all of Earth‘s oceans combined. At the bottom of the huge ocean, hydrothermal vents are suspected, as they are known from Earth. Primitive life could develop there. From 2031 onwards, the mission should provide new insights into the possible basis for extraterrestrial life. It remains exciting.